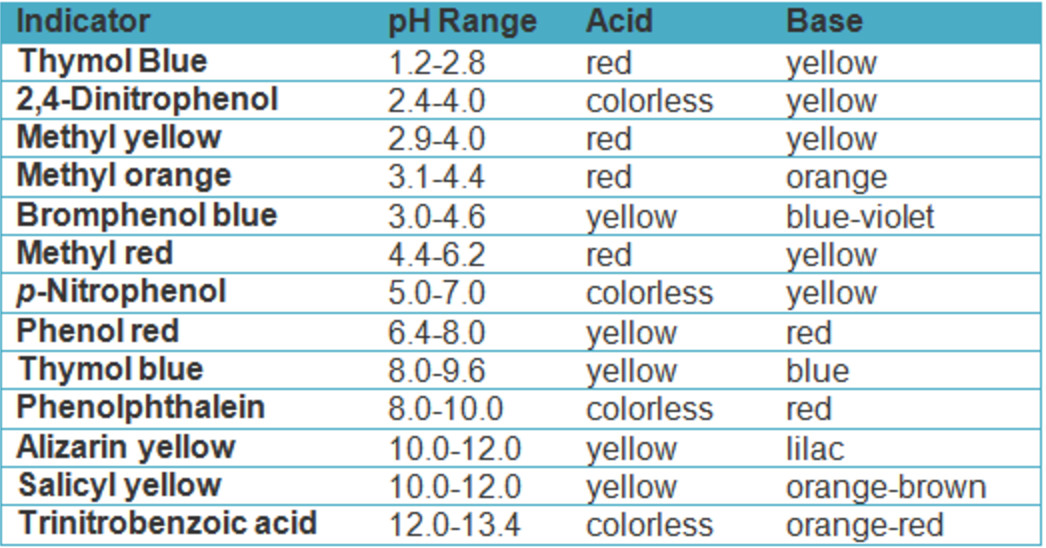
Types Of Indicators In Acid Base Titration . Hcl + naoh → nacl + h 2 o. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are.
from classnotes.org.in
There are two basic types of acid base titrations, indicator and potentiometric. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. Hcl + naoh → nacl + h 2 o. Ph colour indicators are molecules that: The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Types Of Indicators In Acid Base Titration The ph range of phenolphthalein is about 8.3 to 10.0, but the. The ph range of phenolphthalein is about 8.3 to 10.0, but the. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. Ph colour indicators are molecules that:
From www.vrogue.co
Acid Base Titration Setup Phenolphthalein Indicator V vrogue.co Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. In an indicator based titration you add another. Ph colour indicators are molecules that: The ph range of. Types Of Indicators In Acid Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Types Of Indicators In Acid Base Titration A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. There are two basic types of acid base titrations, indicator and potentiometric. Ph colour indicators are molecules that: Hcl + naoh → nacl + h 2 o. In an indicator based titration you add another. The ph range of. Types Of Indicators In Acid Base Titration.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another. Ph colour indicators are molecules that: A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. Hcl. Types Of Indicators In Acid Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Types Of Indicators In Acid Base Titration Hcl + naoh → nacl + h 2 o. In an indicator based titration you add another. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: There are two basic types of acid base titrations, indicator and potentiometric. A) are a weak acid or base (so they react with hydronium) b). Types Of Indicators In Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. The ph range of phenolphthalein is about 8.3 to 10.0, but the. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms. Types Of Indicators In Acid Base Titration.
From mungfali.com
Acid Base Titration Indicator Types Of Indicators In Acid Base Titration In an indicator based titration you add another. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. There are two basic. Types Of Indicators In Acid Base Titration.
From luz-has-richmond.blogspot.com
How Tknow Which Indicator to Use for Titrations LuzhasRichmond Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. The ph range of phenolphthalein is about 8.3 to 10.0,. Types Of Indicators In Acid Base Titration.
From www.shutterstock.com
Acidbase Titration Setup Phenolphthalein Indicator Vector image Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. In an indicator based titration you add another. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Hcl + naoh → nacl + h. Types Of Indicators In Acid Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Types Of Indicators In Acid Base Titration Ph colour indicators are molecules that: There are two basic types of acid base titrations, indicator and potentiometric. Hcl + naoh → nacl + h 2 o. In an indicator based titration you add another. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. The ph range of. Types Of Indicators In Acid Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Types Of Indicators In Acid Base Titration There are two basic types of acid base titrations, indicator and potentiometric. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b). Types Of Indicators In Acid Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Types Of Indicators In Acid Base Titration The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another. Ph colour indicators are molecules that: Hcl + naoh → nacl + h 2 o. There are two basic types of acid base titrations, indicator and potentiometric. A) are a weak acid or base (so they react with hydronium) b). Types Of Indicators In Acid Base Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Types Of Indicators In Acid Base Titration In an indicator based titration you add another. Ph colour indicators are molecules that: The ph range of phenolphthalein is about 8.3 to 10.0, but the. There are two basic types of acid base titrations, indicator and potentiometric. Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b). Types Of Indicators In Acid Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Types Of Indicators In Acid Base Titration A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Hcl + naoh → nacl + h 2 o. The ph range of phenolphthalein is about 8.3 to 10.0,. Types Of Indicators In Acid Base Titration.
From www.slideserve.com
PPT Chapter 15 AcidBase Titrations & pH PowerPoint Presentation ID Types Of Indicators In Acid Base Titration Hcl + naoh → nacl + h 2 o. A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another. Ph colour indicators are molecules that: There are two basic. Types Of Indicators In Acid Base Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Types Of Indicators In Acid Base Titration Ph colour indicators are molecules that: A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. There are two basic. Types Of Indicators In Acid Base Titration.
From www.slideshare.net
Acid base titration Types Of Indicators In Acid Base Titration A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. Ph colour indicators are molecules that: There are two basic. Types Of Indicators In Acid Base Titration.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Types Of Indicators In Acid Base Titration In an indicator based titration you add another. Hcl + naoh → nacl + h 2 o. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. There are two basic. Types Of Indicators In Acid Base Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Types Of Indicators In Acid Base Titration The ph range of phenolphthalein is about 8.3 to 10.0, but the. Ph colour indicators are molecules that: A) are a weak acid or base (so they react with hydronium) b) have conjugate acid and base forms that are. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Hcl. Types Of Indicators In Acid Base Titration.