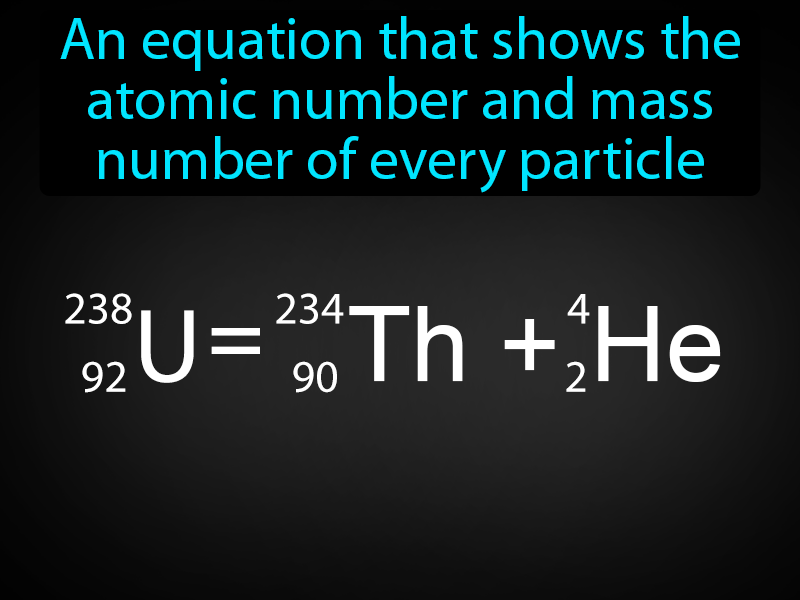What Is Atomic Equation . Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. For an element, relative atomic mass is the average mass of the naturally occurring. Web the average atomic mass (sometimes called atomic weight) of an. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. It is simple to learn how to. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Web atomic mass, the quantity of matter contained in an atom of an element.
from gamesmartz.com
For an element, relative atomic mass is the average mass of the naturally occurring. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Web the average atomic mass (sometimes called atomic weight) of an. Web atomic mass, the quantity of matter contained in an atom of an element. It is simple to learn how to.
Nuclear Equation Definition & Image GameSmartz
What Is Atomic Equation The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web atomic mass, the quantity of matter contained in an atom of an element. It is simple to learn how to. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. For an element, relative atomic mass is the average mass of the naturally occurring. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the average atomic mass (sometimes called atomic weight) of an.
From www.linquip.com
Mastering Nuclear Equation Calculations with Linquip's Expert Guidance What Is Atomic Equation It is simple to learn how to. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Web the average atomic mass (sometimes called atomic weight) of an. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in. What Is Atomic Equation.
From onlinesciencenotes.com
Chemical reactions, balanced and unbalanced chemical equations Online What Is Atomic Equation Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Web the average atomic mass (sometimes called atomic weight) of an. For an element, relative atomic mass is the average. What Is Atomic Equation.
From chem.libretexts.org
11.2 Nuclear Equations Chemistry LibreTexts What Is Atomic Equation For an element, relative atomic mass is the average mass of the naturally occurring. Web atomic mass, the quantity of matter contained in an atom of an element. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Web the average atomic mass (sometimes called atomic weight) of an. Atomic mass. What Is Atomic Equation.
From www.youtube.com
Atomic Structure Formulas / Chemistry Formulas by Basic Class YouTube What Is Atomic Equation It is simple to learn how to. For an element, relative atomic mass is the average mass of the naturally occurring. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is. What Is Atomic Equation.
From joannewong.weebly.com
Chemical Writing & Balanced Equations Upper sec Science What Is Atomic Equation The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the average atomic mass (sometimes called atomic weight) of an. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Web the atomic mass of. What Is Atomic Equation.
From present5.com
AMOUNT OF SUBSTANCE Relative atomic, molecular and formula What Is Atomic Equation Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Web the average atomic mass (sometimes called atomic weight) of an. Web the atomic mass of. What Is Atomic Equation.
From www.slideserve.com
PPT Chapter 21 Nuclear Chemistry PowerPoint Presentation, free What Is Atomic Equation Web atomic mass, the quantity of matter contained in an atom of an element. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. For an element, relative atomic mass is the average mass of the naturally occurring. Web the average atomic mass (sometimes called atomic weight) of an.. What Is Atomic Equation.
From www.showme.com
Balancing Nuclear Reactions Science, Chemistry, Physics ShowMe What Is Atomic Equation It is simple to learn how to. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. For an element, relative atomic mass is the average mass of the naturally occurring. Atomic mass in an atom or group of an atom is the sum of the masses. What Is Atomic Equation.
From www.linquip.com
Nuclear Fission Equation 2 Examples (Practical Guide) Linquip What Is Atomic Equation For an element, relative atomic mass is the average mass of the naturally occurring. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the average atomic mass (sometimes called atomic weight) of an. Web atomic mass, the quantity of matter contained in an atom of an element.. What Is Atomic Equation.
From www.youtube.com
2 Relative Atomic and Formula Mass YouTube What Is Atomic Equation Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is. What Is Atomic Equation.
From socratic.org
What part of an atom is involved in nuclear reactions? Socratic What Is Atomic Equation Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring. What Is Atomic Equation.
From sciencenotes.org
What Is a Chemical Equation? Definition and Examples What Is Atomic Equation Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the average atomic mass (sometimes called atomic weight) of an. It is simple to learn how to. Web the. What Is Atomic Equation.
From sciencetallis.weebly.com
4. Atomic Structure THOMAS TALLIS SCIENCE What Is Atomic Equation Web atomic mass, the quantity of matter contained in an atom of an element. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the average atomic mass (sometimes called atomic weight) of an. It is simple to learn how to. Web the atomic mass of an element. What Is Atomic Equation.
From www.worksheetsplanet.com
What is a Chemical Equation Definition What Is Atomic Equation The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. It is simple to learn how to. Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Web atomic mass, the quantity of matter contained in an atom. What Is Atomic Equation.
From www.slideserve.com
PPT Chapter 7 Chemical Formula Relationships PowerPoint Presentation What Is Atomic Equation Web the atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. Web atomic mass, the quantity of matter contained in an atom of an element. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. Atomic mass in an atom or. What Is Atomic Equation.
From www.slideserve.com
PPT Balancing Chemical Equations 2 PowerPoint Presentation, free What Is Atomic Equation Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance. It is simple to learn how to. Web the average atomic mass (sometimes called atomic weight) of an. Web atomic mass, the quantity of matter contained in an atom of an element. Web the atomic mass of an element. What Is Atomic Equation.
From gamesmartz.com
Nuclear Equation Definition & Image GameSmartz What Is Atomic Equation It is simple to learn how to. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the average atomic mass (sometimes called atomic weight) of an. Web the atomic mass of an element is a weighted average of all the element's isotopes based on their natural abundance.. What Is Atomic Equation.
From studylib.net
Nuclear Equations What Is Atomic Equation It is simple to learn how to. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons. Web the average atomic mass (sometimes called atomic weight). What Is Atomic Equation.